Behind the clouds is the sun still shining;
Thy fate is the common fate of all,
Into each life some rain must fall"-Henry Wadsworth Longfellow
The heart is one of the most complicated organs of the human body and by far one of the most useful. Without the heart, no other organ in our body would function. The heart is solely responsible for pumping oxygenated blood to every single organ, tissue and cell in your body. We now live in an age where we are no longer concerned with starvation but instead morbid obesity (at least in the US). In the past, humans were hunters and gatherers. We were constantly on the move for our next meal and had to stay alert for predators. In this day and age we rarely ever have to drive more than 5-10 minutes to get to our closest fast food restaurant.
As a Paramedic, I get to take care of these individuals when they are going through a medical crisis like having an heart attack, or scientifically known as an Acute Myocardial Infarction (AMI). For some bizarre reason the heaviest of these patients choose to live on the second or third floor of their apartment building. These patients are not only a physical burden but they are also a financial burden to the Medical community at large. Our Medical Community, for the most part, never makes a real issue out of these patients being morbidly obese. What is the reason for biting our collective tongues? We don't want to hurt anyone's feeling by calling them "fat". So, as a morbidly obese patient, when you go to your doctor; they will prescribe you a myriad of antihypertensive/hyperlipidemia/diabetes drugs instead of a strict diet and a gym membership. The obesity epidemic is continuing to grow in the United States at an alarming rate.
According to the AHA's most recent statistics (2013):
- 23.9 million children ages 2 to 19 are overweight or obese. Of these children, 12.7 million are obese.
- Obesity is being defined as an epidemic (a widespread occurrence of a disease in a community at a particular time) in the United States.
- Among Americans age 20 and older, 154.7 million are overweight or obese (Body Mass Index greater than 25). Of these, 78.4 million are obese (BMI of 30 or higher). As of 2012, according to the United States Census Bureau, the US population is 313.9 million.
- According to the National heart forum analysis, the current cost of obesity-related health care costs is a staggering $147 billion.
An analysis conducted by the National Heart Forum estimates that 50 percent of Americans are on track to become obese in the next 20 years. According to the report, 36 percent of Americans are obese. Why am I discussing obesity? The myriad of health problems related to obesity, which include:
- Type 2 diabetes (approx. 25 million Americans)
- Coronary heart disease and Stroke (27 million Americans). There are 795,000 strokes reported per year, as of 2012.
- Obesity-related Cancer, one in there cancer deaths is related to obesity, poor nutrition or physical inactivity. There are approximately 190,650 cases reported per year.
MGuard provides embolic protection in stenting procedures by placing a micron mesh sleeve, which the company calls MicroNet™, over their stent,. InspireMD currently is marketing their stent for use mainly in patients with acute coronary syndromes, notably acute myocardial infarction (heart attack) and saphenous vein graft coronary interventions (bypass surgery).
Before we continue further, let me take some time and explain a little bit of Cardiology. Now I have to warn you, that Cardiology is by no means a simple subject matter but I will try my best to try to explain it as clearly as I can. If you are in the medical field and familiar with Cardiology, you can skip this part or read through for a refresher.
(click to enlarge)
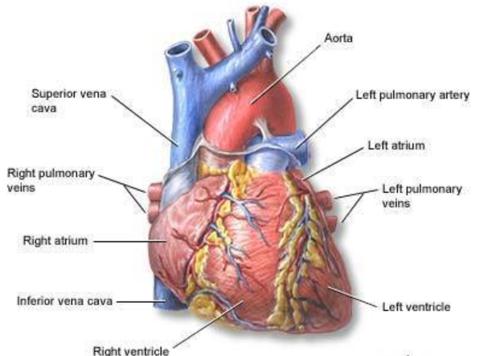
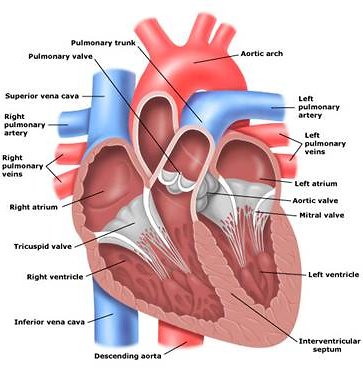
The Human heart and the blood vessels involved.
According to the CDC:
- Approximately 600,000 patients die of heart disease in the United States every year- this is 1 in every 4 deaths. Heart disease is the leading cause of death for both men and women.
- Every year about 715,000 Americans have a heart attack. Of these, 525,000 are a first heart attack and 190,000 happen in patients who already had prior heart attack.
- Coronary heart disease alone costs the United States $108.9 billion each year.
- Diabetes,
- Hypertension (high blood pressure)
- Hypercholesterolemia (high blood cholesterol)
- Hyperlipidemia (an abnormally high concentration of fats or lipids in the blood)
- Obesity
- Cigarette smoking
- Oral contraceptive use
- Poor diet
- Sedentary life style
- Cocaine use, and even
- Stress
- The right atrium
- The left atrium
- The right ventricle
- The left ventricle
Route of blood through the heart
The oxygen depleted blood enters the right atrium through the superior and inferior vena cava. The superior and inferior vena cava are the largest veins that connect back to the heart. Most of this blood passes from the right atrium into the right ventricle via the tricuspid valve. These valves are like a gate between the atria and ventricles that prevents backflow of blood. From the right ventricle, blood is pumped through the pulmonary arteries and into the lungs. While the blood is in the pulmonary circulation, Carbon dioxide is removed and Oxygen is added to the blood. After the blood has been oxygenated in the lungs, the blood goes through four pulmonary veins and into the left atrium. The blood passing from the left atrium to the relaxed left ventricle opens the bicuspid valve. From the left ventricle blood is pumped through the aortic valve and into the aorta. The aorta is the largest artery in the human body. After the blood reaches the aorta, it travels through the systemic circulation reaching smaller and smaller arteries, arterioles, goes to the capillaries, then into venules, veins and back into the superior and inferior vena cava. Then the process for oxygenating blood begins anew.
The Coronary Vessels
The blood within the heart does not supply oxygen and other nutrients to the cells of the heart. The branch of the systemic circulation that supplies the heart is called the coronary circulation. The coronary circulation consists of:
- Coronary arteries- which receive blood through openings in the aorta called the coronary ostia, and
- Cardiac veins- which empty into the right atrium through another ostium (a small opening or orifice), the opening of a large vein called the coronary sinus.
The left coronary artery divides into two main branches:
- The left anterior descending artery (LAD) -delivers blood to portions of the left and right ventricles and much of the intraventricular septum. The intraventricular septum is the wall that separates the ventricles of the heart from one another.
- The left circumflex artery (LCX) - supplies blood to the left atrium, the posterior and lateral walls of the left ventricle and part of the anterior papillary muscle. In 40-50% of the hearts, the LCX supplies the artery to the Sino Atrial (SA) node. The SA node is the "pacemaker of the heart" and sets the rate.
- The Conus, which supplies blood to the upper right ventricle
- The right marginal branch, which traverses the right ventricle to the apex; and
- The posterior descending branch, which supplies smaller branches to both ventricles.
Pathophysiology of Atherosclerosis
Atherosclerosis is a disease process characterized by progressive narrowing of the lumen (inside space of a tubular structure) of medium and large arteries (e.g. coronary arteries). The process results in the development of thick, hard atherosclerotic plaque. Atherosclerosis occurs to some extent in all middle-aged and older patients. Associated risk factors include age, family history of heart disease, cigarette smoking, obesity, hypertension, and diabetes.
Atherosclerosis has two major effects on blood vessels.
- The disease disrupts the innermost lining of the blood vessels. This causes a loss of elasticity in the vessels and increases the formation of clots.
- The plaque reduces the diameter of the vessel lumen. This decreases the blood flow to the tissues and cells.
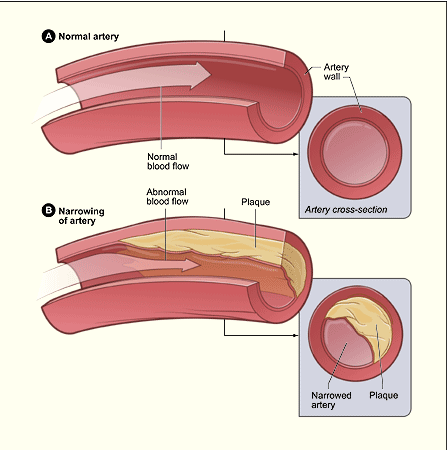 Courtesy www.cardiachealth.org/heart-information/atherosclerosis
Courtesy www.cardiachealth.org/heart-information/atherosclerosisAngina Pectoris
Angina pectoris is a symptom of myocardial ischemia (decreased oxygen supply to the tissues of the heart). The term literally means "choking pain in the chest". Angina occurs in patients when there is an imbalance between myocardial oxygen supply and demand. The ischemia results in a buildup of lactic acid and carbon dioxide in ischemic tissues of the heart. Think about what happens what happens to your muscles when you exercise without rest. The most common cause of angina pectoris is atherosclerotic disease of the coronary arteries. Myocardial ischemia can put patients at risk for cardiac dysrhythmias.
Angina is typically classified as stable and unstable.
- Stable Angina is usually precipitated by physical exertion or emotional distress. The pain usually does not last a very long time and is relieved by rest, nitroglycerin or oxygen. Nitroglycerin is a medication that in essence opens up the blood vessels of the heart and reduces the oxygen demand of the heart.
- Unstable angina, also called pre-infarction angina, is chest pain that can occur during periods of mild physical exertion or even at rest. Patients with unstable angina are at increased risk of an acute myocardial infarction (heart attack) and sudden death. Although majority of patients feel pain in their chest, other patients may describe the pain is in their shoulders, arms, neck, jaw or back. Patients may also present with anxiety, shortness of breath, nausea or vomiting and diaphoresis (profuse sweating).
Acute myocardial infarction occurs with a sudden and total blockage or near blockage of blood flowing through an affected coronary artery to an area of heart muscle. This blockage results in ischemia, injury, and necrosis to the area of the myocardium distal to the occlusion. AMI is most often associated with atherosclerotic heart disease.
AMI typically begins with the formulation of an atherosclerotic plaque involving the intimal (innermost) layer of the coronary artery. The plaque disrupts the smooth arterial lining and results in an uneven surface. This creates turbulent blood flow through the coronary artery/arteries. The plaque may rupture. If the rupture does occur, the injured tissue is then exposed to circulating platelets. This results in the formation of a thrombus that occludes the artery. As the thrombus becomes bigger, it further reduces the blood flow to the coronary vessel.
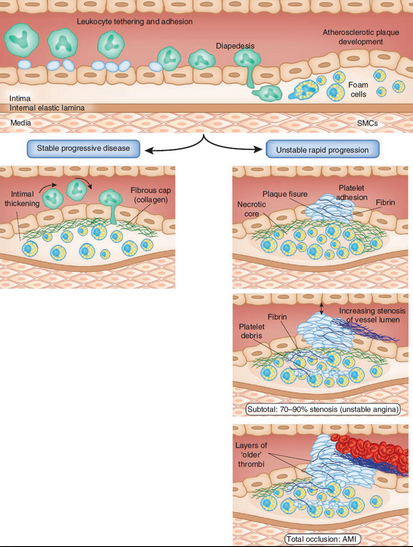
Courtesy http://www.nature.com/nm/journal/v17/n11/fig_tab/nm.2515_F4.html
The next thing to discuss is the Electrocardiography (ECG). Since explaining ECG would take an inordinate amount of time, I will keep it very simple.
(click to enlarge)
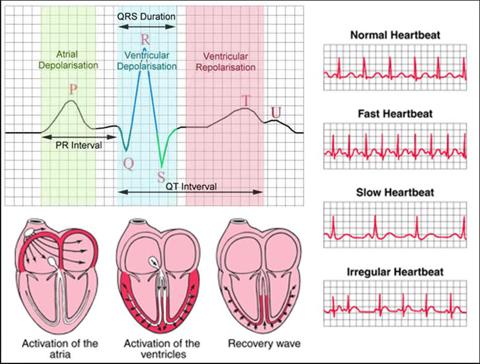
Courtesy http://www.ivline.info/2010/05/quick-guide-to-ecg.html
ECG records the electrical activity of the heart and prints it out for interpretation. All you really need to know is that the ST-segment is elevated in some patients suffering from an AMI and that is why doctors use the term STEMI. Not all patients having an MI will be having a STEMI but all patients with a STEMI are having a heart attack. The device was invented by Dr. Willem Einthoven in 1903 and received the Nobel Peace prize in medicine in 1924 for it.
(click to enlarge)
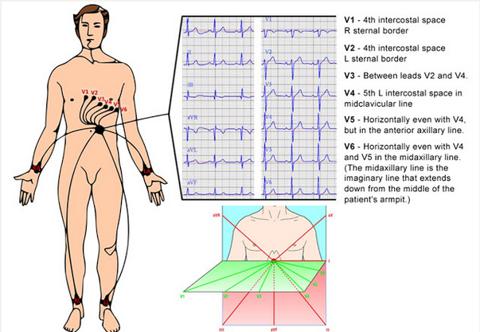
Courtesy http://www.ivline.info/2010/05/quick-guide-to-ecg.html
MGuard provides embolic protection in stenting procedures by placing a micron mesh sleeve over their stent. InspireMD currently is marketing their stent for use mainly in patients with acute coronary syndromes, notably acute myocardial infarction (heart attack) and saphenous vein graft coronary interventions (bypass surgery).
Current stent technology targets the stable angina patient. Stable angina is classified as chest pain from poor blood flow through the coronary blood vessels.
Unstable Angina patients (patients suffering from an acute myocardial infarction) represent a complex clinical problem not addressed by current stent technology.
InspireMD's MGuard reduces mortality rate (measure of the frequency of death), reduces myocardial damage associated with acute myocardial infarction (AMI).
Current standard of care:
Minor heart attack treated with a Bare Metal Stent (BMS) or Drug Eluting Stent (DES)
Causes:
Potential for Debris (plague) to flow downstream, occluding small arteries "Distal Embolization"
Leading to:
Cardiac Mortality and Morbidity
Picture Courtesy InspireMD website:
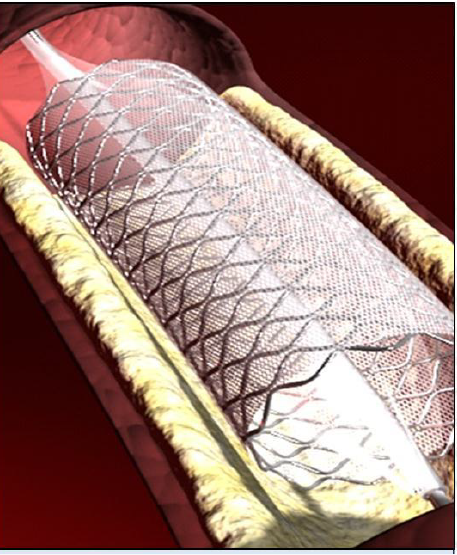
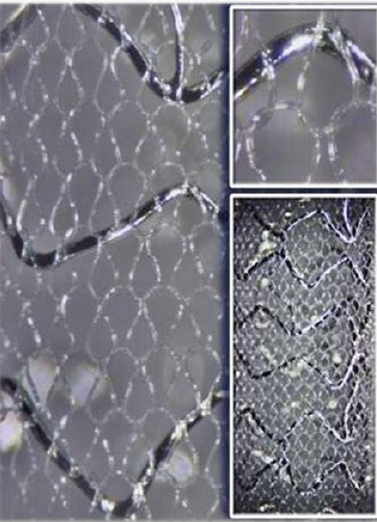
The true genius of the MGuard device is that it combines the stent and embolic protection in a single device. Due to this unique approach, MGuard:
- Reduces risk for embolization by capturing potentially harmful debris, such as plaque, against the artery wall.
- These micro-particles then slowly reenter the artery in a non-harmful way over approximately 30 days.
- The MicroNet on the stent acts as a safety net by providing greater surface area coverage to prevent large debris flow in smaller arteries.
- The proprietary circular knitted mesh wraps around the stent to protect the patient from debris flowing downstream.
- MicroNet is made of a single fiber from a biocompatible polymer, which is widely used in medical implantations today. This material is also very flexible and it does not promote thrombosis (local coagulation or clotting of the blood in a part of the circulatory system)
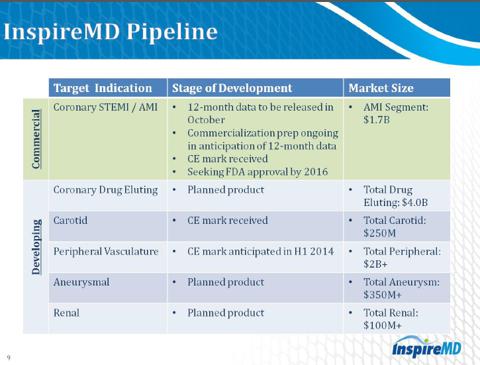
As you can clearly see from the figures above, that a potential standard of care treatment in the Coronary STEMI/AMI market is going to be highly profitable.
When a patient suffers from occlusion of the coronary artery, there are two main choices. Either place a stent or conduct bypass surgery. Stent placement is by far the safest choice. However, when placing stents, the Cardiologist risk dislodging plaque, which could cause thrombus formation or can travel down further and occlude the smaller coronary arteries causing tissue death. The two main types of stents used in the US, the Bare Metal and the Drug Eluting stent do not prevent the unstable plaque from heading downstream and causing blockage and tissue death. InspireMD has the solution to this problem and that is why I believe it is set to become the standard of care in this patient population.
(click to enlarge)

MASTER Trial Highlights
MGuard achieved primary end point
- Superiority in ST resolution- 57.8% vs 44.7%
- Occurred in 1/217 (0.5%) patients with MGuard
- Occurred in 6/216 (2.8%) patients with BMS or DES type stents
- 17.1gm (MGuard) vs 22.3gm (BMS/DES)
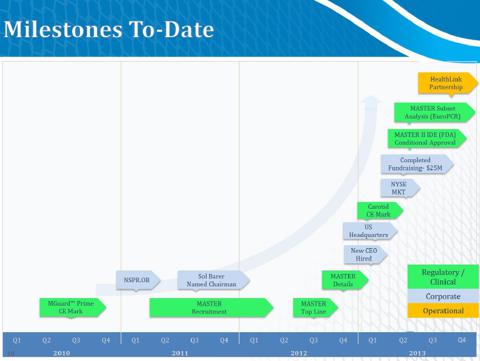 (click to enlarge)
(click to enlarge)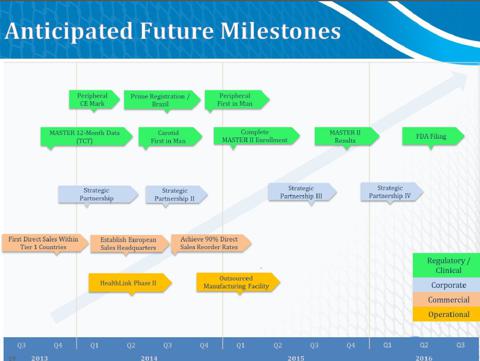
InspireMD (NSPR) some facts to keep in mind:
- The company was founded on February 29, 2008 and is headquartered in Tel Aviv, Israel.
- Market Cap of $98.36 Million (as of 10/05/13), 2.85/share.
- 34.3 Million Shares Outstanding
- NSPR has a 52 Week high of 10.16 and a 52 Week Low of 1.80.
- As of 09/13/2013 has a 0.22% short interest (57,567)
- As of 06/30/2013, the company has 18.27% institutional stock ownership.
- ORBIMED Advisors reported 3,395,000 shares.
- JENNISON ASSOCIATES 1,750,000 shares.
- As of 07/31/13 the company has 5.1% Institutional Mutual Fund ownership
- In the last 12 months, there has been 17 open market buys by insiders. Insider ownership is 10% as reported on 06/30/2013.
- On April 16, 2013 the company diluted shares in a classic biotech fashion to raise funds. NSPR sold 12.5 million shares of its common stock at $2.00 per share. The company received $22.6 million, after deducting underwriting discounts and commissions and other offering-related costs. Following the offering the company does not have any indebtedness for borrowed money outstanding.
- InspireMD currently sells its proprietary stent system technology, MGuard, in 30 countries worldwide.
- InspireMD has 37 patents - 6 granted (1 USA), 31 pending.
- The intellectual property protects key attributes of MicroNet Technology which include anchoring, drug delivery, macro structure and fiber width.
- Pivotal 12-month clinical data to be released Oct 29th, 2013 for the MASTER trial
- NSPR achieved primary endpoint with sustained mortality benefit at 30 days and 6 months
- 12 month MASTER data will provide clinical platform to accelerate sales activities in key international markets.
- Multiple Clinical Trials including the MAGICAL and iMOS studies. Multiple published clinical trails showing the efficacy of MGuard in reducing Major Adverse Cardiac Events (MACE). See resources for a link to those publications.
- Alan Milinazzo is President and CEO. Mr. Milinazzo has worked for Orhtofix, Medtronic, and Boston Scientific.
- Mr. Michael Berman is also part of the team and serving in the capacity of Director. Mr. Berman has an impressive track record. Mr. Berman was the president of the Cardiology Business of Boston Scientific and currently serves on a total of the board of 9 total companies.
- James Barry who was VP, Corporate Research and Advanced Technology Development at Boston Scientific is also a member of the Board.
- This list of other amazing leaders the company has brought on board includes Sol Barer, Campbell Rodgers, Craig Shore, Eli Bar and Chaim Lotan.
Financials
- Revenue for the twelve months ended June 30, 2013, is approximately $4.9 million, a decrease of 0.5 million (8.9%) from the same period in 2012.
- According to the company, the $0.5 million decrease in sales volume was largely due to the fact that the company is in the process of replacing certain third party distributors with direct sales channels. The company believes that this transition to direct selling will ultimately lead to greater sales.
- Gross Profit increased 3.6%, approximately $0.1 million, to approximately $2.6 million from $2.5 million during the same period in 2012.
- Gross margin increased from 46.7% in the 12 month ended June 30, 2012 to 53.2% in the twelve months ended June 30, 2012.
- Research and Development Expenses increased 4.2% or approximately $0.2 million, to approximately $4.2 million, from approximately $4.0 million during the same period in 2012.
- Selling and marketing expenses increased 66.3% or approximately $1.4 million, to approximately $3.6 million, from approximately $2.2 million during the same period in 2012.
- The company believes, with the recent dilution, they have sufficient cash to continue operations into 2015 but may "raise additional funds in 2015 to continue financing" their operations.
- As of June 30, 2103 NSPR had cash and cash equivalents of approximately $14.8 million.
- As of June 30, 2013, NSPR's assets exceeded their liabilities by a multiple of 4.68.
- NSPR repaid $8,787,234 in cash.
I believe that InspireMD is a company with huge potential. At the current share price of $2.85 per share, it is highly undervalued. This is just my analysis of the company but I am not an analyst or a financial advisor. Please do your own DD and never follow investment advice of random people on the internet, including me.
Sources:
http://www.heart.org/idc/groups/heart-public/@wcm/@sop/@smd/documents/downloadable/ucm_319588.pdf
http://www.usnews.com/opinion/blogs/robert-schlesinger/2012/12/28/us-population-2013-more-than-315-million-people
http://healthyamericans.org/assets/files/A.pdf
https://eresearch.fidelity.com/eresearch/evaluate/fundamentals/ownership.jhtml?stockspage=ownership&symbols=NSPR
http://finance.yahoo.com/news/inspiremd-closes-25-million-underwritten-152000063.html
http://biz.yahoo.com/e/130917/nspr10-k.html
http://www.cdc.gov/heartdisease/facts.htm
http://www.ncbi.nlm.nih.gov/pubmed/19937780
http://www.inspire-md.com/site_en/inspiremd-to-sponsor-satellite-symposium-breakfast-meeting-chaired-by-dr-gregg-w-stone-november-9th-at-tct-2011/
(click to enlarge)


